Microwave Assisted Pyrolysis
Microwave-assisted pyrolysis is an energy-efficient and controllable way of converting biomass to chemicals or fuels.
Advantages of MW heating
Microwave-assisted pyrolysis is an energy-efficient and controllable way of converting the feedstock to chemicals or fuels. Microwave heating has a number of advantages over conventional types of heating including the selective activation of various chemicals.
An example of selectivity is biomass pyrolysis. The principal components of the biomass such as hemicellulose, cellulose, and lignin are activated at different temperatures. This makes it possible to increase the selectivity of the bio-oil, collecting different fractions at different temperatures. Moreover, the thermal degradation of polysaccharides proceeds at lower temperatures compared to the conventional methods.
History
Lignocellulosic biorefinery and its latest development are of great importance in economic, environmental and political sectors. There are two main approaches to the activation and utilization of biomass: biochemical (fermentation) and thermochemical (biomass pyrolysis to bio-oil and bio-char). Currently, considerable efforts are being made to increase the scale of biomass transformation.
Alternatively, to the biochemical, the scalability of the thermochemical approach has already been proven by thousand years (from Roman Impair time) production of bio-char and bio-oil. At the middle of the 19th century, the accumulated knowledge allowed getting charcoal at a scale appropriate to satisfy all types of metallurgy while tar cover all demand of shipbuilding. For example, in 1863, the export of tar from Finland reached 28 million liters (170,000 barrels) while the USA was manufacturing nearly 1 million tonnes of biochar per year.
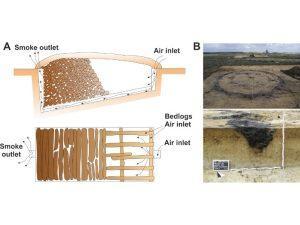
Importance
Under the conditions of increasing worldwide competition at the beginning of 20th century, the cheap coking coal and crude oil have substituted charcoal and tar from their traditional applications. Nowadays issues related to crude oil availability, global warming, and ecological problems are forcing thermochemical methods to make a technological revolution and find a new niche in a global economy.
The success of this strategy could be based on the production of oxygen-containing chemicals. Due to a high degree of functionalisation, these compounds do not need multi-step transformation and are commercially attractive for large industries. Importantly, current industries produce a significant amount of the lignocellulosic waste stream. Only the paper&pulp industry produces 40-50 kg of dry sludge per 1 tonne of paper and subsequently generating municipal solid waste (MSW) containing cellulose polymer as the main constituent. In the EU the problem of MSW has a very high political profile because of its complex character. In the USA there are more than 21 million tons of paper and paperboard waste going to landfill every year.

Challenges
However, ex-situ separation of the oxygen-containing components (once it was produced) is a challenge for the bio-refinery. Even for pyrolysis of cellulose, the simplest biomass constituent, the bio-oil contains more than 100 compounds majority of which are high-boiling, thermally unstable chemicals. Such compounds cannot be separated by distillation. At the same time, efficient separation of the valuable chemicals resulted from cellulose pyrolysis is an essential worldwide task helping to resolve both economic and environmental problems.
Microwave solution and mechanism
The possible solution is in-situ separation and initially selective pyrolysis towards the targeted chemicals or fraction (e.g. charcoal/biochar).
Major biomass structural components are solid oxygen-containing polymers. Microwave irradiation very efficiently interacts with these materials reducing their temperature decomposition compared with conventional pyrolysis. At the same time, most pyrolysis products (except biochar) are gas or volatiles.
Due to the low density of these products, microwave irradiation weakly interacts with them, leaving gas and volatiles compounds at a temperature lowest than during conventional heating. Furthermore, obtained biochar is non-conductive, low-polar, and does not interact with MW irradiation.
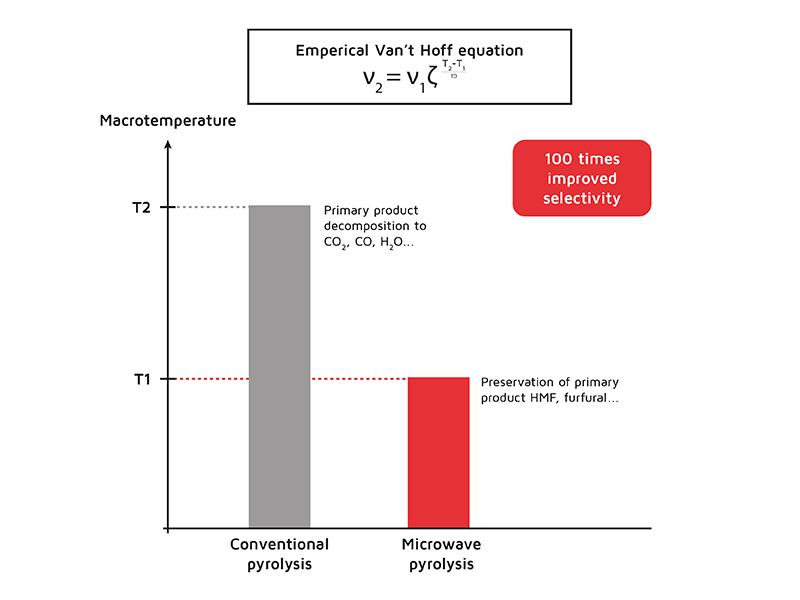
The lowest product temperature in the case of MW heating leads to better selectivity of this process than conventional. The declining selectivity of the main process (pyrolysis) is a result of primary product decomposition. Reducing the main process’s temperature decreases the degradation rate of primary products and therefore declines the selectivity.
Let’s estimate possible selectivity improvement in the microwave process. According to Van’t Hoff’s rule rate of an elementary reaction increases twice every 10 degrees. Literature shows that MW-pyrolysis usually occurs on 80°C lower than conventional. Therefore, the secondary reaction rate in the case of MW pyrolysis will be 28 = 256 times less than in the conventional process. This value is correct only for the initial rate and this effect is decreasing closer to the process end. However, if the resident time of the primary product inside biomass is short, then the selectivity of the process could be improved almost 100 times.
